08 Sep 2020
Withings' ScanWatch – featuring AFib and sleep breathing disturbance detection – is released in Europe
BY Steph Eaves
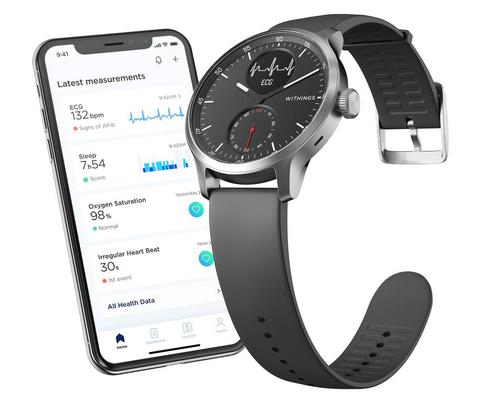
Withings has announced the European availability of ScanWatch – a smartwatch that combines medical-grade electrocardiogram (ECG) and sleep breathing disturbance detection. The wearable will be made available in the US later this year.
The announcement comes after ScanWatch received the CE marking for medical devices. Currently, ScanWatch is CE marked for atrial fibrillation (AFib) detection, both via ECG and photoplethysmography (PPG), and for oxygen saturation (SpO2) measurement. ScanWatch can currently detect overnight breathing disturbances, and is expected to receive the full CE clearance for sleep apnea detection capabilities later this year.
In response to the COVID-19 pandemic and, with it, the rise of telehealth appointments, Withings made the decision to release ScanWatch as early as possible to support consumers and their physicians.
In addition to detecting AFib and overnight breathing disturbances, ScanWatch can also be helpful for those with COVID-19 to monitor their blood oxygen saturation levels on-demand from home via the embedded SpO2 sensor. Withings is currently involved in a research initiative with the Department of Cardiology at the university hospital of the Ludwig-Maximilians University of Munich, which is integrating ScanWatch into a COVID-19 patient monitoring project.
Mathieu Letombe, CEO of Withings, said: “We announced ScanWatch earlier this year to an enthusiastic response. Today, its capabilities to detect heart rhythm disorders as well as to track blood oxygen saturation levels have become even more amplified due to COVID-19.”
The ScanWatch is Withings’ most medically advanced wearable to date. It boasts a battery life of up to 30 days and is designed to help users and their physicians monitor overall health by identifying highly prevalent, yet largely underdiagnosed cardiovascular, respiratory and sleep breathing issues early.
In addition to its AFib and breathing disturbance detection capabilities, ScanWatch includes comprehensive activity tracking, for parameters including steps, calories, elevation, workout routes (via connected GPS) and can automatically recognise more than 30 daily activities such as walking, running, swimming and cycling. It also offers VO2 Max assessments, which is a measure of the heart's and muscles' ability to convert oxygen into energy during physical exercise.
ScanWatch also provides sleep monitoring and analysis of sleep patterns, including the length, depth and quality of sleep, and can wake users up with a gentle vibration at the best time of their sleep cycle.
ScanWatch is commercially available in Europe for €279/£249.95 (38mm) and for €299/ £279.95 (42mm). It will be available in the United States later this year, following FDA-clearance, for $279 (38mm) and $299 (42 mm).
Close Window